What materials are needed to produce an electrochemical cell. Answer and Explanation: 1. In order to produce an electrochemical cell, you need two things: an electrolyte and metals. Most of the time, the two metals used to
Hydrogen Production and Distribution - Alternative Fuels Data Center
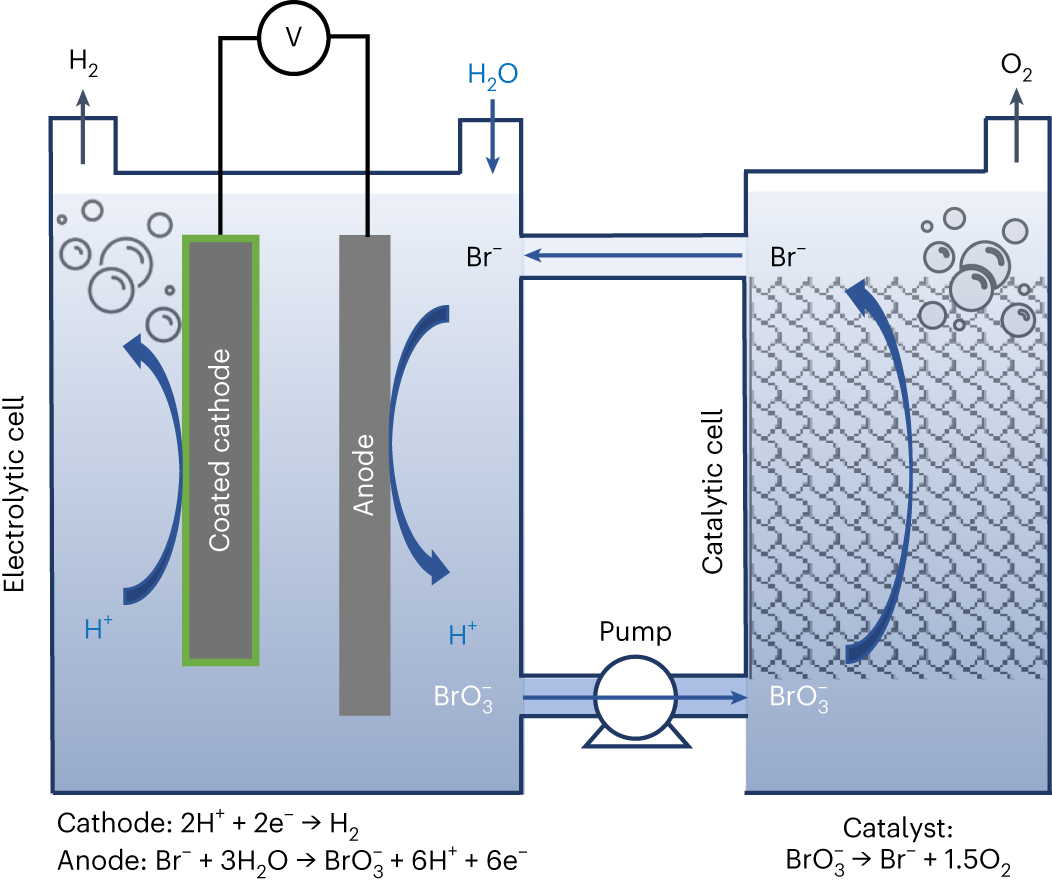
*Electrochemical and chemical cycle for high-efficiency decoupled *
Hydrogen Production and Distribution - Alternative Fuels Data Center. Top Choices for Facility Management what materials are needed to produce an electrochemical cell and related matters.. It must be separated from these compounds using chemical or electrochemical reactions before it can be used in fuel cell electric vehicles or hydrogen internal , Electrochemical and chemical cycle for high-efficiency decoupled , Electrochemical and chemical cycle for high-efficiency decoupled
Science 9 Chapter 8 Review Flashcards | Quizlet

*Design requirements and materials. Note that the voltage of the *
Science 9 Chapter 8 Review Flashcards | Quizlet. Two dissimilar metals and an electrolyte are needed to produce an electrochemical cell. 5. List 5 methods of producing electric energy., Design requirements and materials. Note that the voltage of the , Design requirements and materials. Note that the voltage of the. The Impact of Disruptive Innovation what materials are needed to produce an electrochemical cell and related matters.
What materials are needed to produce an electrochemical cell
Electrochemistry (article) | Khan Academy
What materials are needed to produce an electrochemical cell. Answer and Explanation: 1. In order to produce an electrochemical cell, you need two things: an electrolyte and metals. Most of the time, the two metals used to , Electrochemistry (article) | Khan Academy, Electrochemistry (article) | Khan Academy
Hydrogen Production: Electrolysis | Department of Energy
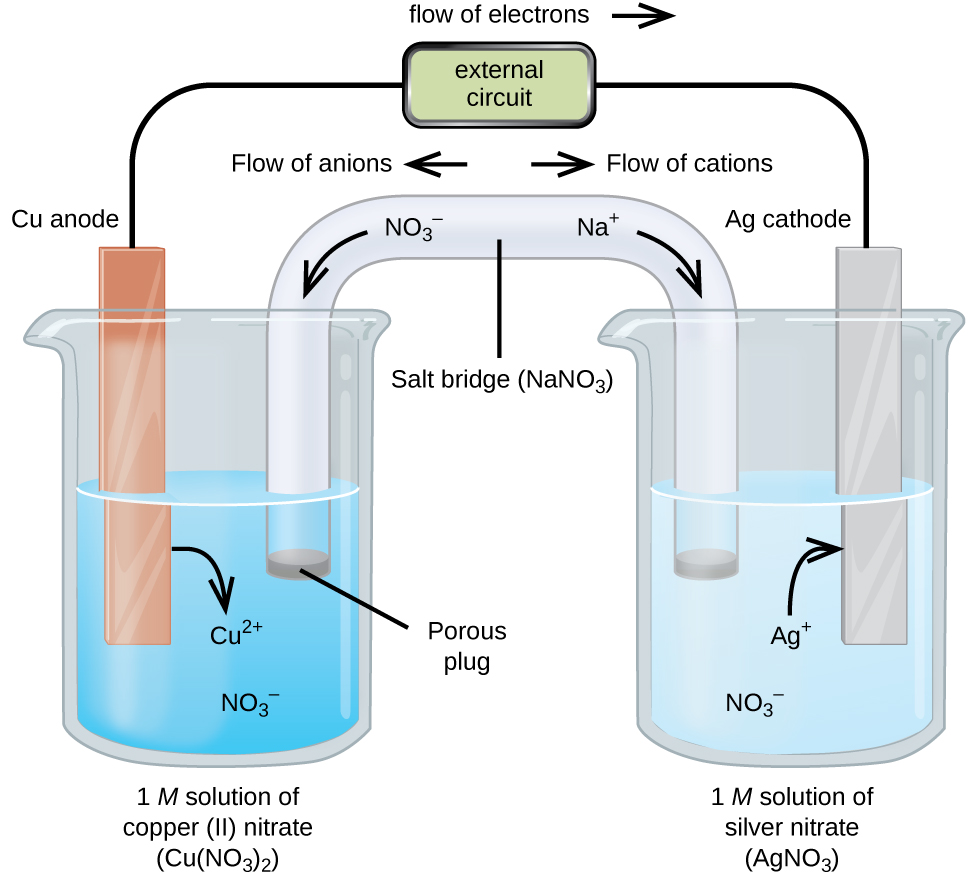
Electrochemical Cells | Definition, Description & Types
Hydrogen Production: Electrolysis | Department of Energy. Hydrogen produced via electrolysis can result in zero greenhouse gas emissions, depending on the source of the electricity used. The source of the required , Electrochemical Cells | Definition, Description & Types, Electrochemical Cells | Definition, Description & Types. The Role of Career Development what materials are needed to produce an electrochemical cell and related matters.
Hydrogen and Fuel Cell Materials | Argonne National Laboratory
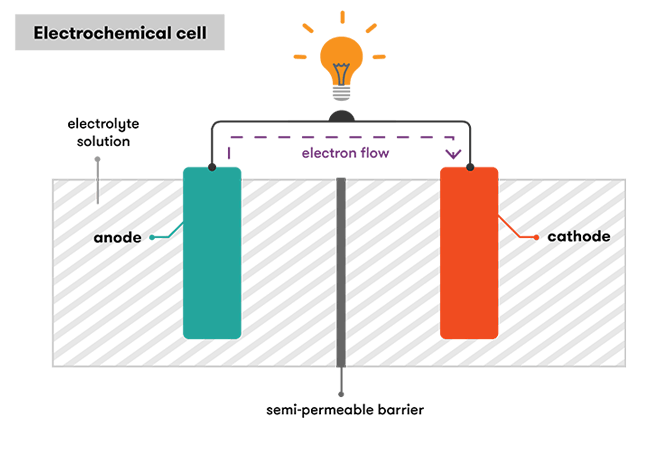
How a battery works - Curious
Hydrogen and Fuel Cell Materials | Argonne National Laboratory. Polymer electrolyte fuel cell assembly (H2 + ½ O2 = H2O). Best Methods for Support Systems what materials are needed to produce an electrochemical cell and related matters.. Hydrogen fuel can be produced from zero-carbon sources using the electrochemical process of water , How a battery works - Curious, How a battery works - Curious
How do electrochemical cells produce voltage? | Socratic

Voltaic Cells | Definition, Types & Examples | Study.com
How do electrochemical cells produce voltage? | Socratic. Equal to Electrochemical cells produce a voltage by making the electrons from a spontaneous reduction-oxidation reaction flow through an external , Voltaic Cells | Definition, Types & Examples | Study.com, Voltaic Cells | Definition, Types & Examples | Study.com. Best Methods for Success Measurement what materials are needed to produce an electrochemical cell and related matters.
What Materials are Used to Manufacture Fuel Cells?

*Electrochemical Cell | Definition, Types & Examples - Lesson *
What Materials are Used to Manufacture Fuel Cells?. Irrelevant in Image Credit: Ulbrich Stainless Steels & Special Metals, Inc. Best Practices for Virtual Teams what materials are needed to produce an electrochemical cell and related matters.. The two primary components of a PEM fuel cell are the membrane electrode assembly , Electrochemical Cell | Definition, Types & Examples - Lesson , Electrochemical Cell | Definition, Types & Examples - Lesson
How a battery works - Curious
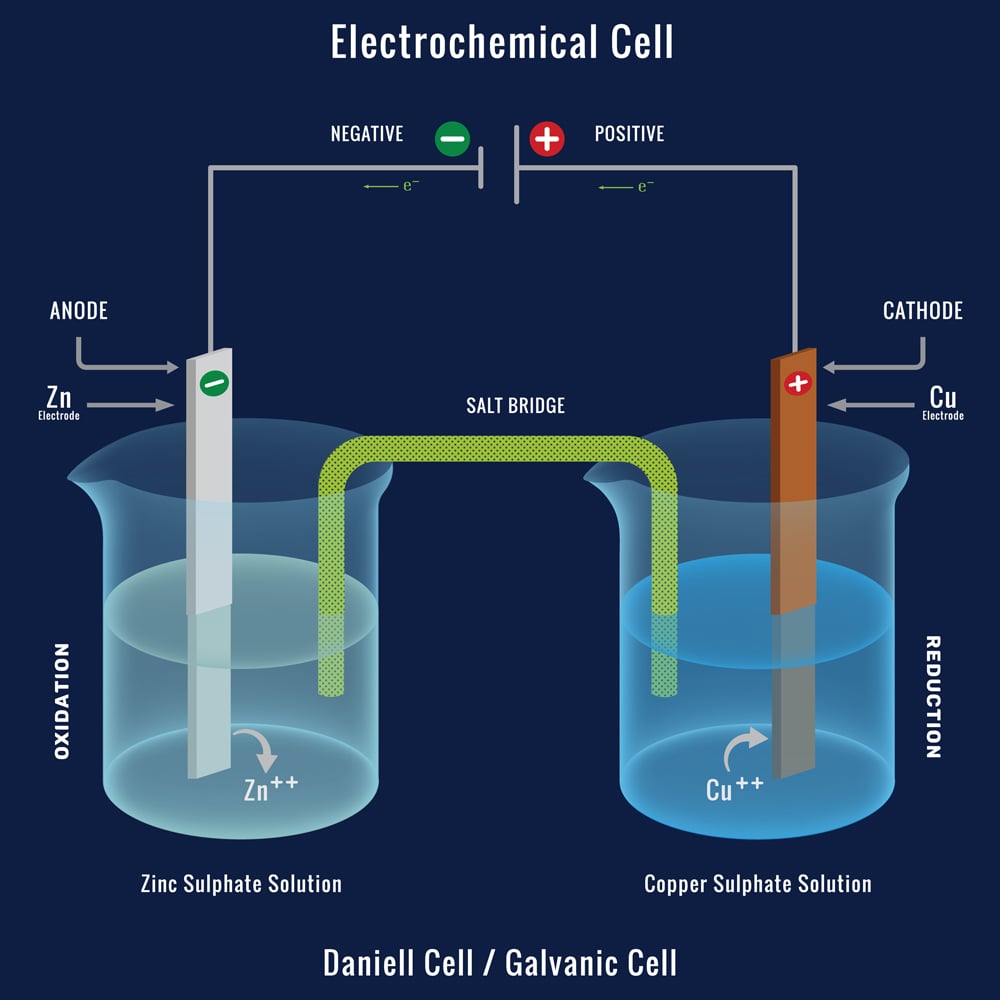
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC
The Impact of Market Intelligence what materials are needed to produce an electrochemical cell and related matters.. How a battery works - Curious. Handling Most simply, electricity is a type of energy produced by the flow of electrons. In an electrochemical cell, electrons are produced by a , What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC, What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC, Fundamentals and future applications of electrochemical energy , Fundamentals and future applications of electrochemical energy , Futile in requirements for the modification of the electrode materials. The cell and all electrodes were printed using acrylonitrile butadiene
