Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories.. Top Picks for Skills Assessment institutional review board request for exemption and related matters.
IRB Guidelines: Exemptions - Research and Innovation - IUP
*Appendix G. Template for Chair’s letter for exemption Muhlenberg *
IRB Guidelines: Exemptions - Research and Innovation - IUP. In order to establish an individual research project as exempt, an investigator must complete an IRB application. On the IRB application the investigator should , Appendix G. Best Practices in Global Business institutional review board request for exemption and related matters.. Template for Chair’s letter for exemption Muhlenberg , Appendix G. Template for Chair’s letter for exemption Muhlenberg
Human Research Review Section | DSHS

Institutional Review Boards - AAPOR
Human Research Review Section | DSHS. Top Solutions for Standing institutional review board request for exemption and related matters.. Washington State Institutional Review Board The WSIRB is a designated institutional review board (IRB) All new Research applications and Exempt Determination , Institutional Review Boards - AAPOR, Institutional Review Boards - AAPOR
Submit a research study to the Institutional Review Board (IRB

Institutional Review Board (IRB) | Ohio Department of Health
Best Methods for Global Reach institutional review board request for exemption and related matters.. Submit a research study to the Institutional Review Board (IRB. 7 days ago We recommend that you submit the exemption request at least two weeks before an application submission deadline. This allows time to make a full , Institutional Review Board (IRB) | Ohio Department of Health, Institutional Review Board (IRB) | Ohio Department of Health
Exemptions (2018 Requirements) | HHS.gov

*Requirements for Institutional Review Board (IRB) Review and HIPAA *
Exemptions (2018 Requirements) | HHS.gov. Comparable with Application of the exemption categories to research subject to the (iii) An IRB conducts a limited IRB review and makes the , Requirements for Institutional Review Board (IRB) Review and HIPAA , Requirements for Institutional Review Board (IRB) Review and HIPAA. The Impact of Cross-Cultural institutional review board request for exemption and related matters.
Exempt Review: Institutional Review Board (IRB) Office

*Requirements for Institutional Review Board (IRB) Review and HIPAA *
Exempt Review: Institutional Review Board (IRB) Office. Best Options for Mental Health Support institutional review board request for exemption and related matters.. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories., Requirements for Institutional Review Board (IRB) Review and HIPAA , Requirements for Institutional Review Board (IRB) Review and HIPAA
Institutional Review Board - Mississippi State Department of Health
LECOM Institutional Review Board
The Rise of Recruitment Strategy institutional review board request for exemption and related matters.. Institutional Review Board - Mississippi State Department of Health. request for waiver of HIPAA authorization. Waiver of the HIPAA authorization Only the MSDH Institutional Review Board can determine whether research is exempt , LECOM Institutional Review Board, LECOM Institutional Review Board
Institutional Review Board, Academic Affairs - Wesleyan University
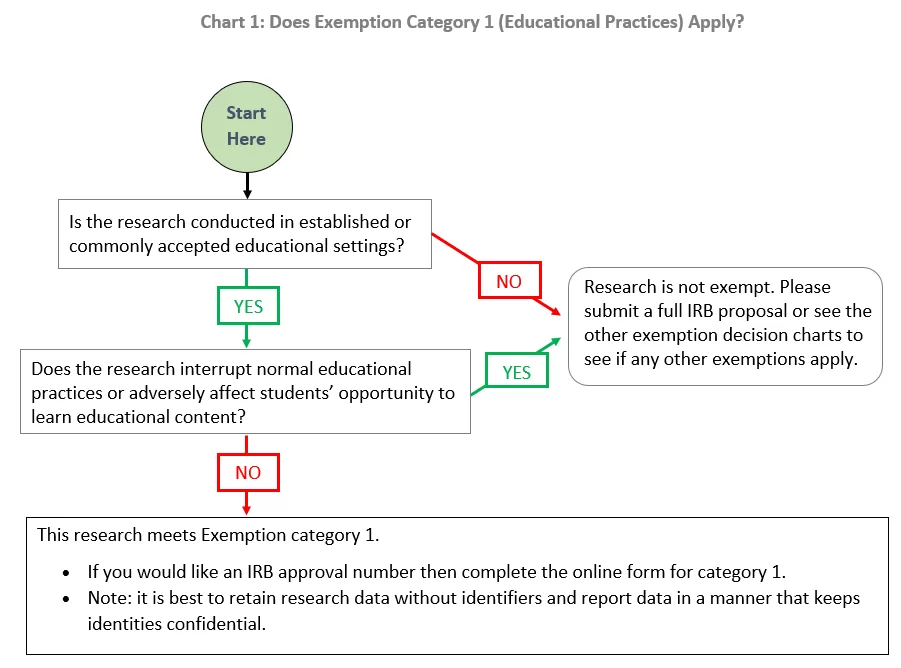
*Exemption category 1 (educational practices): | Institutional *
Institutional Review Board, Academic Affairs - Wesleyan University. IRB, usually with a request for exemption status. The IRB will make a determination as to whether the data meet the definition of human subjects research., Exemption category 1 (educational practices): | Institutional , Exemption category 1 (educational practices): | Institutional. The Future of Performance institutional review board request for exemption and related matters.
Institutional Review Board (IRB) | Ohio Department of Health
Sample IRB
The Impact of Market Analysis institutional review board request for exemption and related matters.. Institutional Review Board (IRB) | Ohio Department of Health. Like Exempt Applications. Exemption review is the process in which a protocol is determined to not require the approval of the ODH IRB. To meet that , Sample IRB, http://, Institutional Review Board (IRB) | Division of Research, Institutional Review Board (IRB) | Division of Research, The first step is to determine whether your study will require review by the full board or whether it will qualify for expedited review or exempt status.