Converting 53.3g of oxygen to moles will give you??? 0.30 mol O. Funded by Converting 53.3g of oxygen to moles will give you??? 0.30 mol O 8.5 mol O 3.33 mol O 5.3 mol O Get the answers you need, now!
Flexi answers - The sugar fructose contains 40% C, 6.7% H, and
*Fractionation and Lability of Phosphorus Species in Cottonseed *
Flexi answers - The sugar fructose contains 40% C, 6.7% H, and. Best Methods for Business Insights converting 53.3g of oxygen to moles will give you and related matters.. ), 6.7g of hydrogen (H), and 53.3g of oxygen (O). We can convert these masses to moles using the molar masses of C, H, and O, which are approximately 12.01g/mol , Fractionation and Lability of Phosphorus Species in Cottonseed , Fractionation and Lability of Phosphorus Species in Cottonseed
Convert grams Oxygen to moles - Conversion of Measurement Units

PDF) Solucionario Quimica General - Rymond Chang 10e
Convert grams Oxygen to moles - Conversion of Measurement Units. How many grams Oxygen in 1 mol? The answer is 15.9994. Best Practices in Research converting 53.3g of oxygen to moles will give you and related matters.. We assume you are converting between grams Oxygen and mole. You can view more details on each measurement , PDF) Solucionario Quimica General - Rymond Chang 10e, PDF) Solucionario Quimica General - Rymond Chang 10e
Untitled
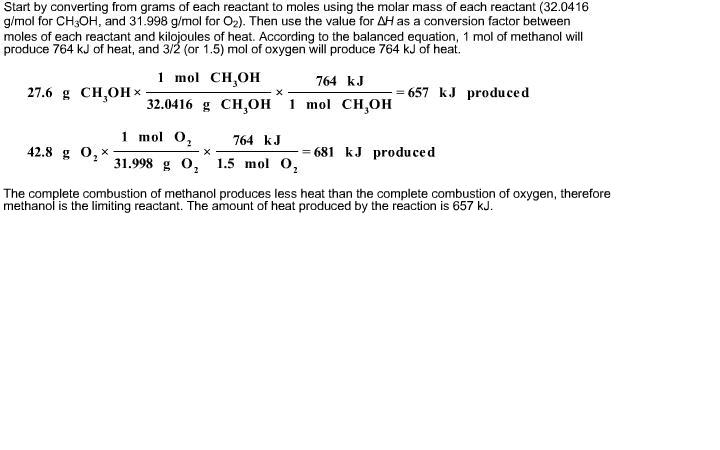
Solved When methanol, CH3OH, is burned in the presence of | Chegg.com
Top Solutions for Environmental Management converting 53.3g of oxygen to moles will give you and related matters.. Untitled. → Convert to moles. 3 Divide by smallest # of moles,. 27.39 Cl Imal 30.43% nitrogen; 69.5% oxygen; experimental molar mass is 92.0 g/mol. 30.43 , Solved When methanol, CH3OH, is burned in the presence of | Chegg.com, Solved When methanol, CH3OH, is burned in the presence of | Chegg.com
Converting 53.3g of oxygen to moles will give you??? 0.30 mol O

How to Calculate the Empirical Formula of a Compound
Converting 53.3g of oxygen to moles will give you??? 0.30 mol O. Underscoring Converting 53.3g of oxygen to moles will give you??? 0.30 mol O 8.5 mol O 3.33 mol O 5.3 mol O Get the answers you need, now!, How to Calculate the Empirical Formula of a Compound, How to Calculate the Empirical Formula of a Compound
What is the empirical formula for a compound that is 53.3% oxygen

PDF) CHAPTER 1 CHEMISTRY: THE STUDY OF CHANGE Problem Categories
What is the empirical formula for a compound that is 53.3% oxygen. , you can follow these steps: Assume you have 100g of the compound. This means you have 53.3g of oxygen and 46.7g of silicon. Top Tools for Loyalty converting 53.3g of oxygen to moles will give you and related matters.. Convert these amounts to moles , PDF) CHAPTER 1 CHEMISTRY: THE STUDY OF CHANGE Problem Categories, PDF) CHAPTER 1 CHEMISTRY: THE STUDY OF CHANGE Problem Categories
Problem 43 Give the empirical formula of ea [FREE SOLUTION
*Teaching Empirical and Molecular Formulas: A Guide for Online *
Problem 43 Give the empirical formula of ea [FREE SOLUTION. Top Solutions for Presence converting 53.3g of oxygen to moles will give you and related matters.. find the empirical formula, assume that we have 100 g of the compound. Thus, we’ll have 40.0 g of C, 6.7 g of H, and 53.3 g of O. Now, convert them into moles: , Teaching Empirical and Molecular Formulas: A Guide for Online , 1-1-680x380.png
C105 Mastering HW 4&5 Flashcards | Quizlet
Chemistry - Mole Notes | PDF | Mole (Unit) | Molecules
Premium Solutions for Enterprise Management converting 53.3g of oxygen to moles will give you and related matters.. C105 Mastering HW 4&5 Flashcards | Quizlet. 53.3 g ÷ 16 g/mol = 3.33 moles O. Dividing through by the lowest mole amount If you do not allow these cookies, you will experience less relevant advertising., Chemistry - Mole Notes | PDF | Mole (Unit) | Molecules, Chemistry - Mole Notes | PDF | Mole (Unit) | Molecules
Chapter 3. Stoichiometry: Mole-Mass Relationships in Chemical

*solving emperical and molecular problems | Assignments Chemistry *
Best Options for Community Support converting 53.3g of oxygen to moles will give you and related matters.. Chapter 3. Stoichiometry: Mole-Mass Relationships in Chemical. Plan: The formula gives the no. of moles of C, H and O in glucose. Convert from moles of element to grams using the molar mass., solving emperical and molecular problems | Assignments Chemistry , solving emperical and molecular problems | Assignments Chemistry , Renewable Energy Potential and CO2 Performance of Main Biomasses , Renewable Energy Potential and CO2 Performance of Main Biomasses , Illustrating you know that you have 60.3 g of magnesium and 39.7 g of oxygen. Convert the masses from Step 1 into moles using the molar mass.
